Pipeline

Specific cell targeting in vivo: right cell, right payload, right disease
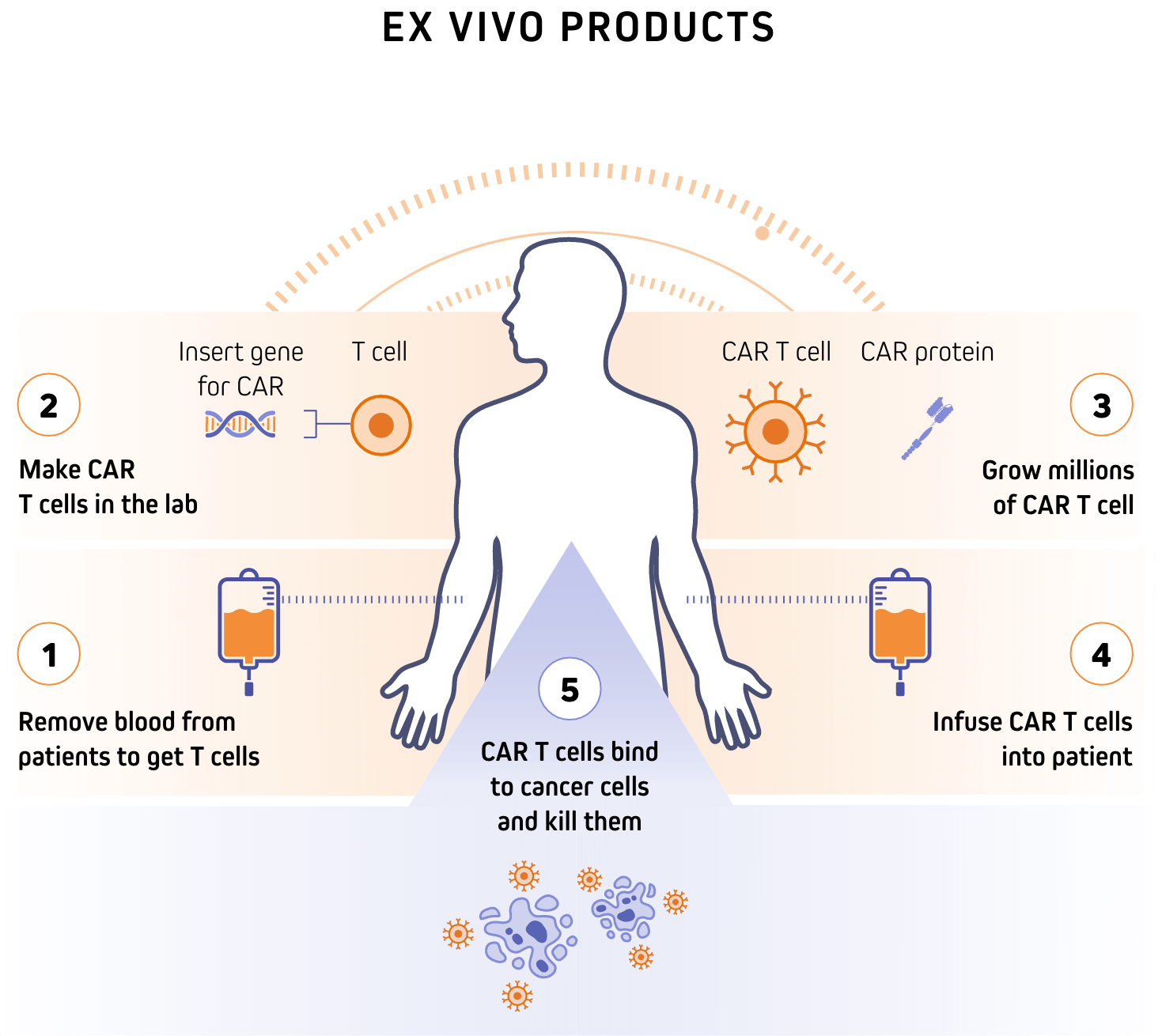
The first application of our platform is aimed at overcoming the current challenges faced in the ex vivo chimeric antigen receptor (CAR) T-cell therapy field, including toxic chemotherapy of patients, long wait times due to manufacturing bottlenecks, high cost, and procedure complexity that impair access. Our platform’s ability to re-engineer cellular function in situ has yielded a pipeline of therapeutic candidates that offer best-and-first-in-class potential for the treatment of B-cell malignancies and severe autoimmune diseases.
Our platform’s unique ability to generate highly targeted CAR T and NK cells in vivo has the potential to greatly expand the CAR therapy market, reaching patients who are currently unable to receive these therapies in the U.S. and globally.
A pipeline of direct-to-patient targeted genetic medicines
PROGRAM
INDICATION
VECTOR TARGET
Discovery
IND-ENABLING
Phase 1
INT2104
B cell malignancies
T and NK cells
Discovery
IND-ENABLING
Phase 1
INT2106
Severe autoimmune
T and NK cells
Discovery
IND-ENABLING
Phase 1
INT2108
Undisclosed
Undisclosed
Discovery
IND-ENABLING
Phase 1
Reinventing CAR therapy to expand therapeutic reach
Through the precise delivery of the CAR gene payload, facilitated by our engineered lentivector platform, our lead candidate INT2104 can be administered directly via IV infusion without preconditioning chemotherapy, aiming to improve patient accessibility and limit off-target side effects. INT2104 has been designed to generate CAR-T and NK cells directly in vivo, bypassing the need for costly and time-consuming ex vivo cell manipulations.
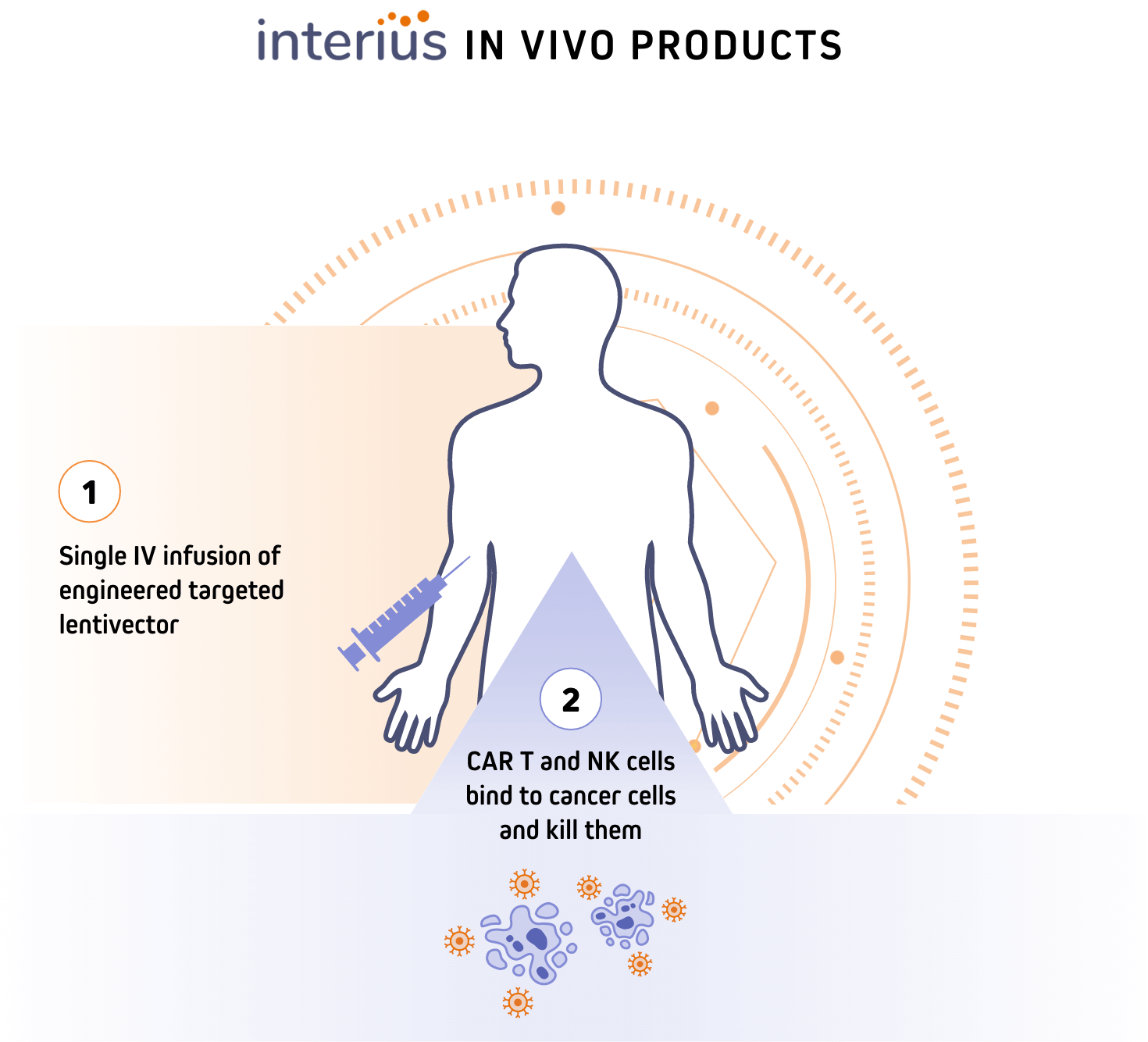
How Interius’ in vivo products work
Engineered lentivector specifically delivers CAR gene to T and NK cells in the body
Reprogrammed CAR T and CAR NK cells bind to B cells and kill them
INT2104
Our lead candidate targeting CD7 offers best-in-class potential for treating B-cell malignancies in a single intravenous (IV) infusion. The INT2104 product creates CAR T cells and CAR NK cells in the patient’s own body. These cells in turn seek out and destroy CD20-expressing malignant B cells. Preclinical data support the validity of this approach, with complete depletion of human B cells and tumor elimination in humanized mouse models after a single IV dose. In addition, INT2104 has demonstrated biologic function and safety in a formal GLP toxicology study in nonhuman primates.
INT2106
Leveraging our platform targeting T and NK cells, and all the benefits of eliminating ex vivo cell manipulation in CAR therapy, this candidate offers best-in-class potential to induce immune reset in patients with a range of serious autoimmune diseases that are mediated by the production of autoantibodies. The INT2106 product leads to the creation of CAR T and CAR NK cells in the patient’s own body with specificity to eliminate CD19-expressing B cells, which are the culprit in producing autoreactive antibodies in numerous autoimmune indications. Preclinical studies have demonstrated complete depletion of human B cells in humanized mouse models, and GLP toxicology data support the safety of the drug candidate.
INT2108 and beyond
Our third pipeline program is currently undisclosed, but it is based on our platform, leveraging valuable experience from prior programs to facilitate development. In addition, we continue to work on researching new concepts and potential drug candidates that are applicable to various genetic medicine applications in diseases in which pathology can be mediated by the expression of a gene of interest in specific tissues. For any indication our platform will provide convenient IV administration of an off-the-shelf biologic in wide-ranging patient care settings, on-target specificity of genetic cargo delivery, manufacturing at scale with a lower cost of goods, and the fulfillment of our vision to democratize genetic medicine. We are driven by making genetic medicine a reality for everybody.
Clinical trials
Learn more about our INT2104 clinical trial, which is currently enrolling participants.