Making genetic medicine a reality for everybody
We engineer targeted, programmable vectors for the precision delivery of genetic medicines to treat an array of challenging diseases.

Our proprietary LENTIVECTOR platform
for precision delivery of genetic medicines
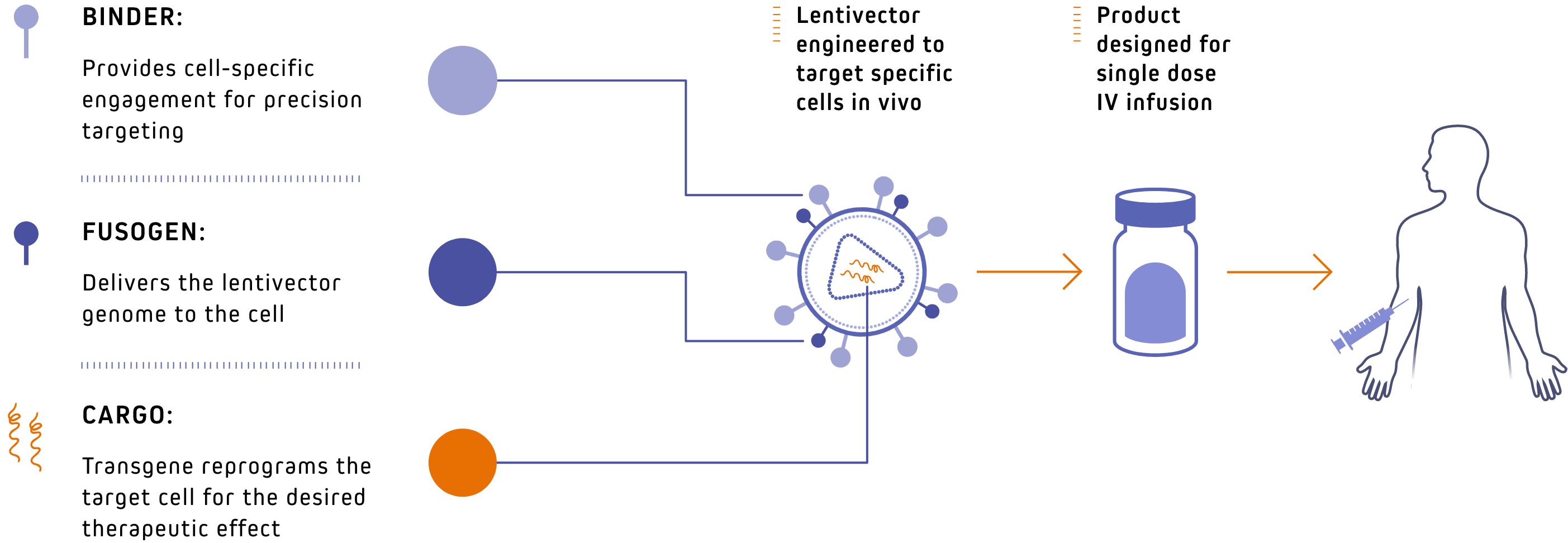
A pipeline with best-in-class potential
Our pipeline of novel lentivector therapeutics harvests multidisciplinary knowledge to bring new options to patients in oncology and autoimmunity.
Where vision meets expertise
We are creating a new generation of genetic medicines with the potential to bring best-in-class therapeutics to patients in need.
Join our
fantastic voyage
We are a tight-knit, data-driven, highly motivated group of professionals on a mission to democratize genetic medicine. Learn how you can join us and become an Interian.


“Being part of the Interius team truly feels like a family. Everyone’s opinions and thoughts matter, and we all have the same goal to grow with the company and make life-changing discoveries.”
Jeffery Foss
Associate Scientist

“Interius is the most diverse company I have worked for. There are so many women from different backgrounds and countries all working together and supporting each other. I have never experienced that professionally, and it gives me the feeling that we as women can do anything put in front of us.”
Tiffany Scott
Accountant
“It is exciting to be part of the passionate Interius team developing breakthrough in vivo cell & gene therapy products to address unmet medical needs and improving accessibility.”
BABU MEDI
SENIOR DIRECTOR OF DRUG PRODUCT DEVELOPMENT
News
See recent media coverage and press releases.