Platform

Our Story: From urgency to innovation
Our story began at the bedside in a hematology-oncology ward at the University of Pennsylvania. Our founder, Dr. Saar Gill, was treating patients with CAR-T therapies and had grown increasingly frustrated with how hard it was to offer these treatments to his patients. The therapies had to be made bespoke for every patient, taking weeks to prepare off-site. Sometimes, they did not arrive back at the clinic in time to treat severely ill patients whose condition worsened while waiting.
Genetic medicine 2.0: Applying clinical experience to deliver better therapies, faster
To answer this front-line clinical need, we founded Interius with the intent to “democratize” genetic medicine. We invented a new type of treatment — a platform — which could be used in multiple indications while offering a “faster, better, cheaper” way to address patient needs.
Our products are rationally designed to offer efficacious treatment, and we are now beginning to test them in the clinic. Our proprietary lentivector platform has been engineered as single-dose, off-the-shelf genetic medicines that can be administered to patients via intravenous infusion and, when used to administer therapies like in vivo CAR-T, without the need for pre-conditioning chemotherapy. Our products are built to supply large indications and are designed to be administered in broad care settings, making the therapies available to every patient in need.
Our genetic medicines can be manufactured at scale, at a cost much lower relative to existing genetic therapies, and can be sent to the pharmacy to wait for the patient, not the other way around.
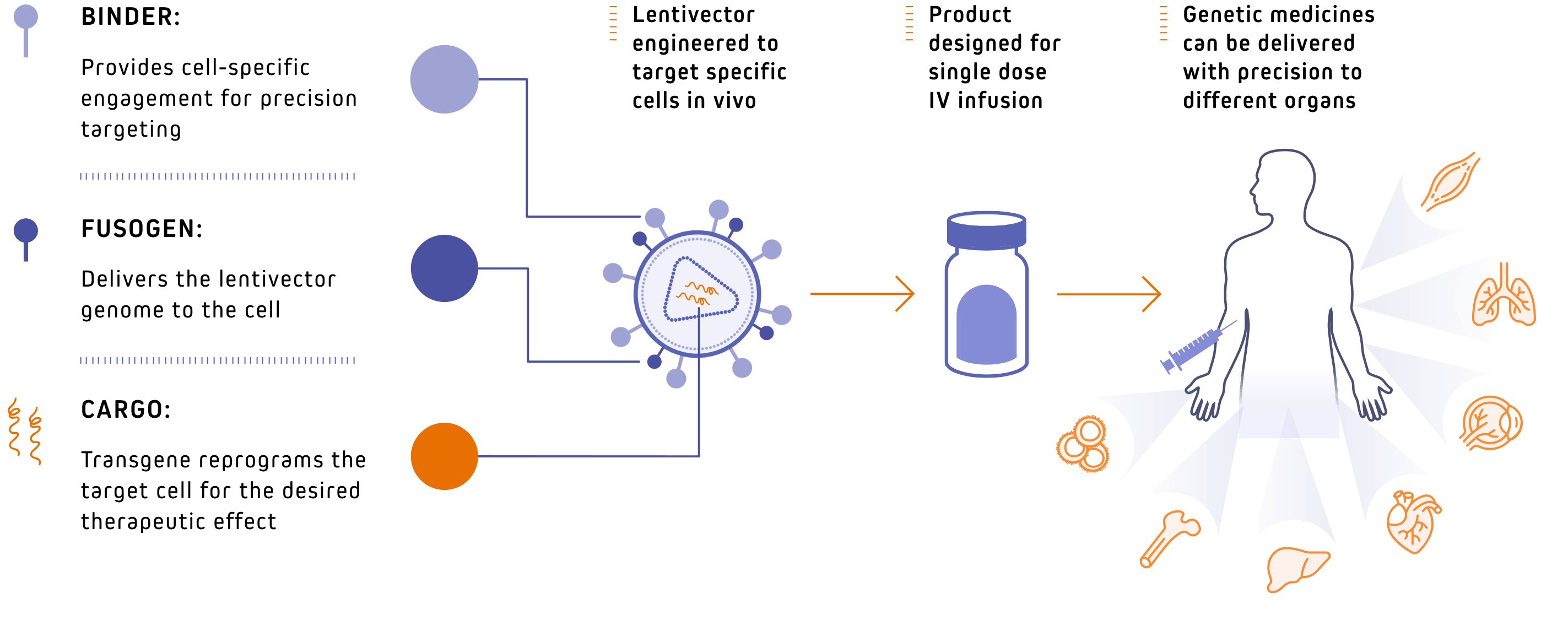
Delivering the right payload to the right cell to fight disease
Rationally designed to be fully programmable, our platform utilizes 3 key components that enable broad application across an array of therapeutic areas and healthcare settings. Custom binders and cargo offer rapid ability to redirect new products for new indications. The particle and cell fusion function has been designed to be independent from target binding, bolstering specificity engendered by the binder.
OUR MODULAR VECTOR DESIGN HAS THREE KEY FEATURES:
Learn more about the research backing our approach by clicking the link below.
Platform advantages
Our platform is designed to make genetic medicines broadly and immediately accessible to patients with cancer, autoimmune conditions, and other serious diseases. Interius’s medicines are intended to be delivered via a single dose intravenous infusion, offering convenience and ease of use to patients and their care teams. Our platform was designed with commercial manufacturing in mind, such that scale and standard processes can reduce the cost of goods and supply the medicines to large indications.
Our initial pipeline products deliver targeted vectors, which create chimeric antigen receptor (CAR) cells directly in the patient’s body. Our vectors eliminate the need to treat patients with toxic chemotherapy ahead of CAR treatment. In addition, by eliminating the need to harvest and modify a patient’s own cells, genetic medicines leveraging our platform can be delivered to patients faster than ex vivo cell therapies. This is especially valuable for patients with rapidly progressing diseases like cancer who cannot afford to wait.
Off the shelf biologic
IV INFUSION
Single dose
SPECIFIC TARGETING
No chemotherapy
SCALABLE MANUFACTURING
Our platform: from clinical problem to clinical solution
- Early 2021: Series A closes
- Late 2021: Interius platform technology invented
- Early 2022: animal models show specificity and biologic effect
- Late 2022: manufacturability of productS demonstrated
- Late 2023: first Interius patent granted
- Late 2023: Formal toxicology studies show clean data
- Late 2023: GMP drug product manufactured
- Early 2024: first product regulatory submission
- Late 2024: first product regulatory clearance
- Q4 2024: first study participant DOSED
From platform to proof of concept
Discover how our platform is enabling us to build a pipeline of therapeutic candidates to overcome unmet needs in the delivery of genetic medicines.